Abcuro is advancing first-in-class programs in autoimmunity and cancer in which highly cytotoxic immune cells play a critical role.
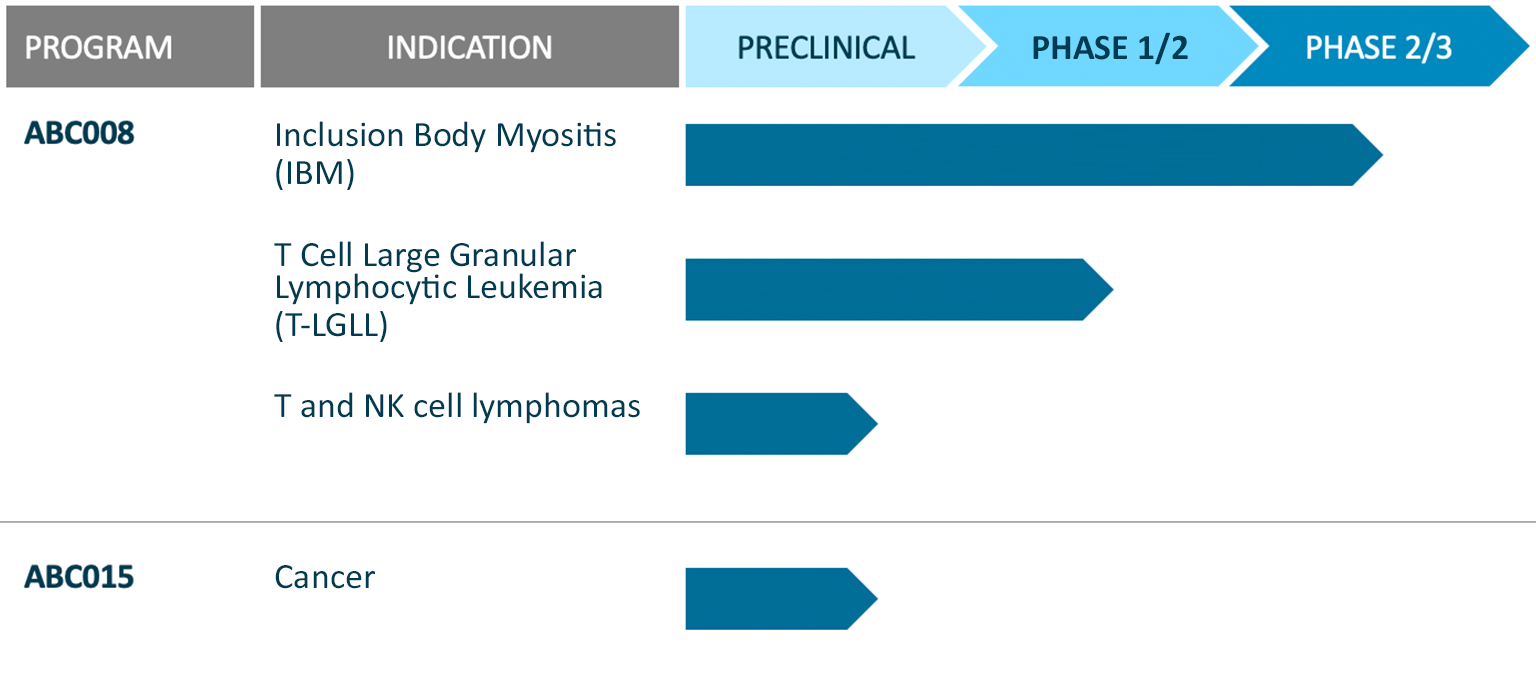
ABC008 is a first-in-class anti-KLRG1 antibody capable of selectively depleting highly cytotoxic T cells, while sparing naïve, regulatory and central memory T cells. ABC008 has been designed to treat diseases mediated by highly cytotoxic T cells, including the autoimmune muscle disease inclusion body myositis (IBM), T cell large granular lymphocytic leukemia (T-LGLL), and mature T cell malignancies.

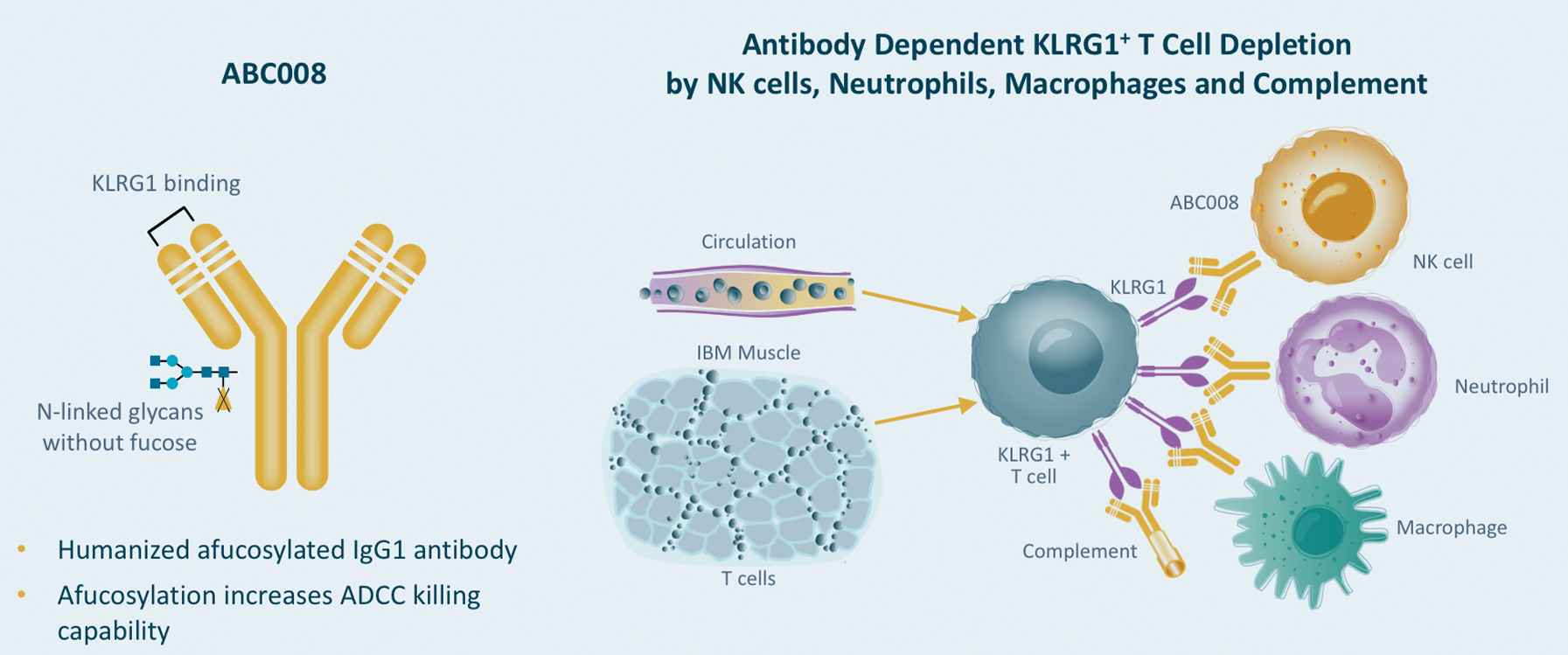

Inclusion Body Myositis (IBM)
Inclusion body myositis (IBM) is the most prevalent myopathy in adults older than age fifty, with over 50,000 patients estimated in the United States and Europe. This debilitating disease is characterized by progressive weakness and atrophy of muscles of the arms and the legs, as well as muscles involved in swallowing. IBM is chronically progressive and results in severe disability and premature death.
IBM is an autoimmune condition in which highly cytotoxic T cells attack muscle which leads to degeneration of the tissue. Muscle tissue from patients with IBM shows the presence of highly differentiated, KLRG1+ cytotoxic T cells destroying muscle fibers.
There are currently no available therapeutic options for IBM. Non-specific immunosuppressants or corticosteroids have not demonstrated efficacy in IBM and thus, are not widely used.
T Cell Large Granular Lymphocytic Leukemia (T-LGLL)
T cell large granular lymphocytic leukemia (T-LGLL) is an autoimmune disorder characterized by clonally expanded CD3+CD8+ cytotoxic T lymphocytes attacking neutrophils and red blood cell precursors, leading to neutropenia and anemia. Neutropenia causes frequent infections, which are a major cause of premature death in patients with T-LGLL. Anemia results in transfusion-dependence in approximately one third of patients.
Given the lack of approved therapeutic options for T-LGLL, about 50% of patients utilize off-label therapies such as the non-specific immunosuppressant methotrexate. Limited efficacy of current standard of care is reflected in an overall reduced life expectancy for T-LGLL patients.
T and NK Cell Lymphomas
T and NK cell lymphomas are a group of aggressive cancers in the lymph and blood that tend to have poor outcomes for patients. The cancer cells are cytotoxic, well-differentiated and can express KLRG1 as a cell surface marker. Patients with these cancers are treated with powerful cytotoxic agents which have limited sustained efficacy and significant side effects. Abcuro aims to assess the efficacy and safety of ABC008, a targeted depleter of cytotoxic T cells and NK cells that express KLRG1 in patients with this group of severe diseases.
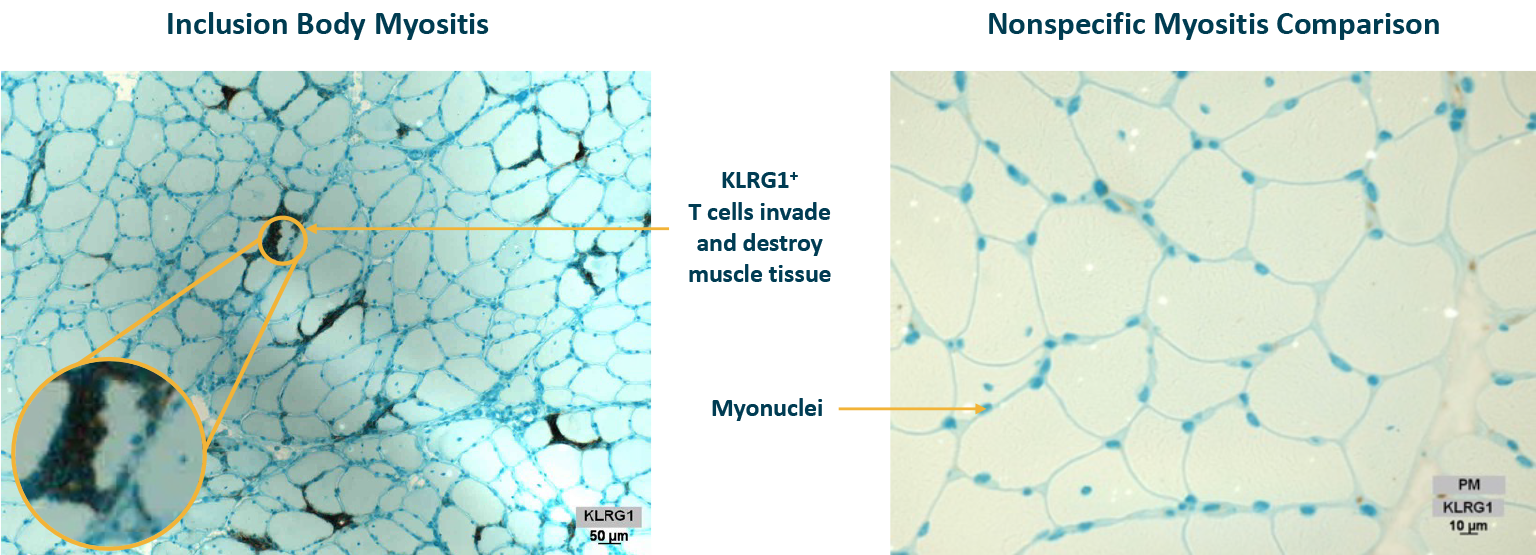
ABC015 is a monoclonal antibody product candidate designed to interrupt the interaction of KLRG1 with E- and N-cadherin, its cognate ligands found on the surface of cancer cells.
Blocking KLRG1, an inhibitory immune checkpoint receptor, on mature T and NK cells, from binding to its ligands on cancer cells could allow these immune cells to more effectively kill cancer cells.

In comparison to PD-1, KLRG1 has superior attributes such as expression on the most potent highly cytotoxic lymphocytes, including the most differentiated T cells and NK cells.

REFERENCES:
ABC008
- Pereira et al., The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity, MAbs 2018 Jul;10(5):693-711
IBM
- Shelly et al., Epidemiology and Natural History of Inclusion Body Myositis: A 40-Year Population-Based Study, Neurology 2021 May 25;96(21):e2653-e2661
T-LGLL
- Shah et al., A population-based study of large granular lymphocyte leukemia, Blood Cancer J, 2016 Aug 5;6(8):e455
- Dinmohamed et al., Population-based analyses among 184 patients diagnosed with large granular lymphocyte leukemia in the Netherlands between 2001 and 2013, Leukemia, 2016 Jun;30(6):1449-51


